
Methyl acrylate entered the global chemical scene in the early twentieth century, when breakthroughs in synthetic chemistry created a path for new industrial polymers and specialty liquids. The discovery of acrylic acid and its esters became a defining moment for the plastics and textiles industries. Factories began producing methyl acrylate at scale, driven by mounting demand for adhesives and coatings during periods of growth and reconstruction. This substance gave chemists a novel building block, and its commercial story tells of the constant interplay between innovation, market demand, and health awareness. Methyl acrylate’s presence in consumer products—from fabrics to glues—often goes unnoticed, but without it, everyday conveniences would face new hurdles.
Methyl acrylate serves as a key monomer for many acrylic polymers and resins. This clear, colorless liquid has a sharp, pungent smell, a warning sign of its volatile nature. Industry values it for its reactivity, as it quickly links with other molecules under the right conditions. Factories pipe it straight into mixing vessels for batch or continuous production. Paper coatings, textiles, and automotive parts rely on methyl acrylate for flexibility and durability. Businesses count on it for rapid curing in adhesives. Its role in practical manufacturing dwarfs the attention it receives in the public eye, but its influence runs deep in many materials we handle each day.
At room temperature, methyl acrylate remains a flammable liquid, boiling at around 80°C and freezing near -75°C. Its density hovers around 0.95 grams per cubic centimeter, and its vapor pressure exceeds 60 mmHg at 20°C, confirming the need for careful storage. Methyl acrylate dissolves in most organic solvents but resists mixing with water; this property shapes its processing, shipping, and handling. A reactive double bond at the heart of each molecule makes it an attractive target for chemical modification. Factories take advantage, pushing it through controlled reactions to build larger, more complex structures.
Chemical plants produce methyl acrylate meeting strict technical standards. Labels must include purity levels, usually above 99%, and list inhibitor content—typically hydroquinone—to prevent runaway polymerization during transit. Storage containers call for hazard diamonds and signal words, a sign of health and environmental risks. Packaging often involves drums lined with special coatings to guard against corrosion and contamination. Transport documents require precise chemical identification numbers, reflecting the lessons learned from decades of mishaps and regulatory evolution.
Industries synthesize methyl acrylate by reacting acrylic acid with methanol in a process known as esterification. In my time working with research teams in basic chemical labs, the importance of temperature and acid catalyst control became clear—overheating or imprecise timings led to reduced yields or dangerous byproducts. Large-scale manufacturers also recover unreacted methanol and recycle it, conserving resources and cutting costs. Few methods offer the same simplicity paired with such impressive output.
Once synthesized, methyl acrylate can undergo radical polymerization, the same reaction that built the past century’s plastics revolution. A single spark—like benzoyl peroxide or even ultraviolet light—starts a chain reaction. The product ends up as a long chain, or polymer, useful in coatings and fibers. Chemists take it further, tweaking side groups or blending in other comonomers to fine-tune product hardness, flexibility, and chemical resistance. At one stage, our small lab mixed methyl acrylate with ethyl acrylate and saw dramatic shifts in the toughness of the resulting plastic, a trick that keeps material scientists busy in search of the next best combination.
Methyl acrylate gets labeled in several ways: 2-propenoic acid, methyl ester; acrylic acid methyl ester; and MAA. Across regions, it shows up as Methylpropenoate, EINECS 202-500-6, or simply as part of product codes in factories that mix it into latexes or paints. This patchwork of names can confuse buyers, new technicians, and regulators—keeping an eye on supplier paperwork and regional safety sheets prevents nasty surprises. Product branding sometimes hides the raw chemical behind trade names, especially in specialty adhesives and paints.
Working around methyl acrylate sharpens everyone’s respect for safety guidelines. The vapors can irritate the eyes and lungs, and skin contact causes burns. Standard safety data sheets urge gloves, goggles, and robust ventilation during handling. Fire departments see it as a hazard, since spills ignite quickly. Manufacturers invest in vapor detectors and emergency response plans. In my own rough early days working in a pilot plant, poorly ventilated rooms and worn seals meant headaches and near misses until investment in modern engineering controls. Global regulations mandate detailed training and continual review of storage systems, minimizing the risk of accidental exposure or fires.
Industries channel methyl acrylate into a wide range of uses. Acrylic fibers owe their strength to this monomer. Auto makers use copolymers for coatings and sealants. Water treatment plants depend on specialty polymers that trap particles as they pass through filters. The textile realm grabs methyl acrylate for colorfast printing. Even the cosmetics sector explores it for certain specialty resins. From printing inks in local community newspapers to adhesives in global packaging, its reach is often hidden in plain sight. Personal experience shows that small tweaks in the chemical structure support new product launches, especially when durability and weather resistance top the requirements.
Labs around the world keep searching for smarter ways to use and modify methyl acrylate. Scientists have found that blending it with renewable feedstocks can cut reliance on fossil fuels, a goal that matters more as resources tighten and climate goals set new limits. Researchers hunt for catalysts that work at lower temperatures, promising cost savings at large scale. During collaborations with graduate programs, the pursuit of new co-monomers that impart flame retardance or anti-bacterial features has led to surprising prototypes, attracting interest from both established brands and startups.
Toxicology studies highlight dangers that call for careful industrial deployment. Methyl acrylate’s vapors irritate the respiratory tract and eyes at low concentrations. Skin contact frequently leads to chemical burns, and prolonged exposure increases the risk of nerve damage. Occupational safety rules insist on monitoring airborne concentrations, setting exposure limits to avoid chronic health effects. Community health experts raise valid concerns about leaks and spills affecting air quality or groundwater. Animal studies point to DNA interactions at higher doses, pushing laboratories to search for safer derivatives. Workers with extended tenure in plants that use these chemicals share personal accounts of skin sensitivity and respiratory discomfort, connecting research findings with on-the-ground realities.
Methyl acrylate faces rising scrutiny from regulators and environmental advocates, yet it still powers much of modern materials science. Companies invest in greener synthesis routes and push recyclable acrylics to market. New research focuses on bio-based alternatives and closed-loop production, aiming to reduce waste and lessen environmental impact. Markets in Asia and the Americas continue to expand as demand for lightweight, durable materials grows. As technologies mature, methyl acrylate could either evolve toward safer, renewable sources or see rival chemistries compete for its staple roles. Staying engaged in research and policy talks gives industry players a realistic shot at shaping a healthier, cleaner future for both workers and consumers.
A lot of talk about chemicals and raw materials gets drowned in technical jargon. Methyl acrylate definitely falls into that pit. On paper, it’s just another clear, sharp-smelling liquid. In reality, it powers a huge slice of modern life. People cross paths with products born from methyl acrylate nearly every day, whether they realize it or not.
I remember trying to fix a broken piece of plastic on my son’s toy car. The glue in my toolkit promised a strong, flexible bond. Turns out, that potion probably owed a lot to methyl acrylate. This chemical forms the backbone of many adhesives, especially the ones that need to hold tight under bending and pulling. Without it, you get brittle glues that crack under pressure.
Many flexible plastics—think clear packaging films, synthetic rubbers in gaskets, and elastic fibers—rely on methyl acrylate too. Manufacturers use it as a building block for crafting plastics that stretch but don’t snap, hold their shape, and weather age better than the plastics from my childhood. The tire industry and sportswear brands depend on it to bring out certain properties in their products. Even paints that resist scratching and cleaning chemicals benefit from its toughness and flexibility.
An honest look at methyl acrylate should factor in its risks. Breathing this chemical at work can cause eye and lung irritation. I met a plastics worker a few years ago who shared stories about improved air handling and strict training that made his workplace safer. He remembered the days before regulations tightened—painful eyes, runny noses, and not many answers from management. Today, safer production sites rely on proper ventilation, smart handling, and frequent monitoring.
Regulators continue pressing companies to track chemical exposure, keep storage areas secure, and train workers. Numbers from the U.S. Occupational Safety and Health Administration drive home that better protocols mean fewer cases of chemical illness. It’s a reminder that protecting people in the lab or factory matters as much as the final product rolling off the line.
The world isn’t about to stop needing flexible plastics or tough adhesives. For me, the real conversation revolves around greener chemistry. For years, industry leaders have looked for safer substitutes and more efficient production systems. Some chemical engineers now focus on recycling methods that recover methyl acrylate from discarded plastic, instead of always making new batches from scratch. Universities and research groups study new bio-based starting points—like sugars from plants—as a possible future for the sector.
Nobody claims it’s easy to swap out a chemical that holds so many different uses. Progress takes real cooperation from factories, regulators, and buyers. But the shift matters. Greener manufacturing processes cut down on pollution and lower risks for both workers and the public.
Methyl acrylate sits behind much of what we touch: tape, paint, furniture coatings, rubber soles, even some cosmetics. People enjoy useful products, but it pays to know how raw materials shape those goods. The story of methyl acrylate shows how a single chemical can straddle the worlds of health and industry, sparking both opportunity and responsibility.
Methyl acrylate does a lot of heavy lifting in the world of plastics, paints, and adhesives. In my own years working near labs and chemical plants, I’ve watched people underestimate how something as common as this liquid can turn risky in a hurry. The big problem isn’t just its strong odor or how fast it evaporates—it’s also about what your body faces if you get careless. Even a splash or a lungful without any barrier can leave burns or set off wheezing fits. That’s no surprise when you look up the Material Safety Data Sheet and see “toxic if inhaled.”
Not a week goes by on any plant floor without reminders about methyl acrylate’s flammability. Vapors can spread wide and light up from a spark across the room. I’ve seen fire drills get real tense because of chemicals just like this. Old habits like using your phone or plugging things in near an open drum quickly get corrected, sometimes with harsh words from folks who’ve seen the aftermath of a chemical flash.
PPE isn’t just for show. If you’re handling methyl acrylate, chemical goggles, a sturdy lab coat, and gloves built for chemical work make a true difference. Nitrile lasts a lot longer than latex here. Sometimes, folks skip the face shield, but I’ve watched a lab veteran avoid a nasty splash with just that extra step. In small operations, people sometimes think it’s overkill, but I trust the gear right alongside folks at a major polymer plant.
Ventilation keeps a lot of trouble away. Good labs use fume hoods and exhausts to keep vapors from building up. I once watched an old hood catch almost all the vapor from a beaker, while a friend nearby with a cup on a bench started feeling lightheaded in minutes. Good air flow steers those clouds away from your mouth and nose. Even if the area smells fine, that doesn’t mean the air is safe. Monitors that track vapor levels help, and supervisors usually scan reports for any spikes.
Methyl acrylate likes to move fast along floors if there’s a spill. I saw speedy cleanup teams roll out absorbent pads and neutralizers before anything could reach a drain. Never once did they go in without full gear or skip the call to plant safety. Containers stay grounded to drain away static before pouring, and lids always get tightened straight after use. Storage stays in metal cans with good seals in cool, ventilated corners, well out of the sun.
I’ve noticed the safest teams are the ones that practice. Emergency showers and eyewash stations aren’t just for show—they land right at the top of checklist drills. People walk routes with their eyes closed, counting steps from their stations. Repeat training means everyone knows what to do, not just in theory, but with muscle memory. Important too, everyone on a safety team knows who to call and what info to pass along if something goes wrong. Quick action needs clear thinking, and drills train for that.
Lab coats, gloves, training, and awareness—skip any one of those and even a familiar liquid like methyl acrylate turns treacherous. My experience tells me: treat it with the respect it deserves, and the workday usually ends without incident. People stay healthy, and nobody has to write up an accident report or call home early. In the long run, it’s less hassle to do things right from the start.
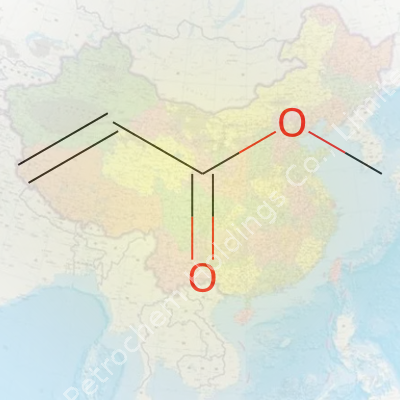
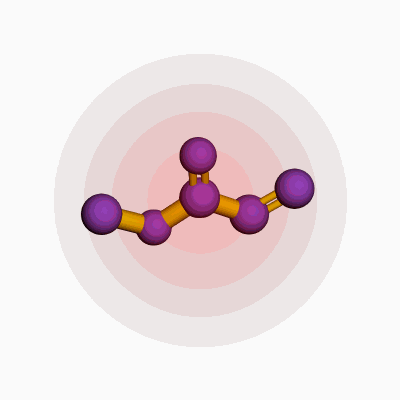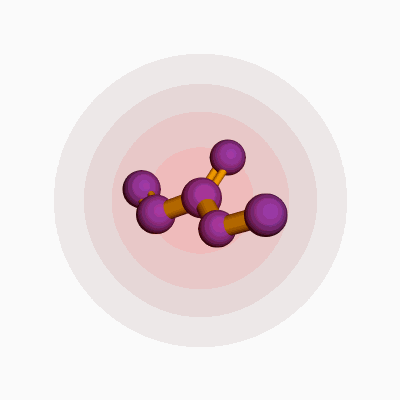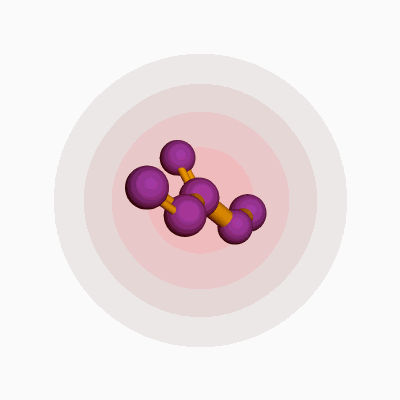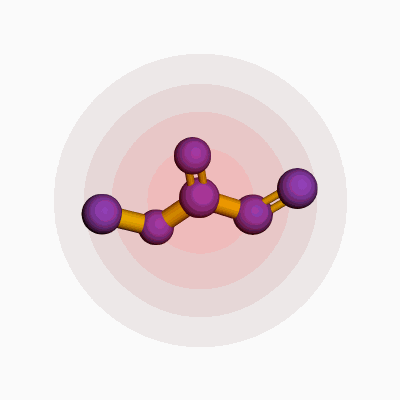
Methyl acrylate, known in the world of chemistry by its formula C4H6O2, shows up on the labels of more products than people often realize. Its molecular structure features four carbon atoms, six hydrogens, and two oxygen atoms. The way these atoms connect—simple yet revealing—offers more insight than a line of textbook jargon. In practice, methyl acrylate belongs to the ester group, sitting between the worlds of organic chemistry and industrial application.
Looking at the skeletal formula, the backbone is pretty straightforward: CH2=CHCOOCH3. Lay it out, and you see why methyl acrylate stands out. On one side, there’s a double bond between two carbon atoms, which chemists call the vinyl group. Off to the other end, an ester group forms from the conjugation of a carboxylic acid and a methanol molecule. This configuration isn’t random. That double bond carries a lot of chemical energy, making the molecule pretty reactive, especially in polymerization processes.
Students and professionals get told to pay attention to chemical structures early because these little drawings can predict so much about behavior. In methyl acrylate’s case, that double bond makes it reactive enough to serve as building blocks for plastics and adhesives. Without it, none of the high-performance resins or flexible coatings industry relies on would exist in the same way.
I’ve worked with manufacturers who rely on materials like methyl acrylate for everyday products—paints, textiles, superabsorbent polymers. The reasons always trace back to that specific layout of atoms. For example, the way the ester group holds up against water helps finished materials keep their shape instead of breaking down. The double bond in the carbon skeleton means the molecule can link up with others during polymerization, creating tough plastic films or binders.
Numbers from the American Chemistry Council estimate that over 100,000 metric tons move through supply chains each year, much of it ending up in construction, packaging, and medical products. This widespread use brings both opportunity and complication. With such demand, safety and environmental impact become pressing concerns. Methyl acrylate carries health hazards—vapors can irritate lungs and eyes, and prolonged exposure needs careful management in workplaces.
Safe handling depends on knowledge and technology. Factories implement closed-system processing and ventilation to limit exposure. Workers wear personal protective equipment. Monitoring air quality and temperature keeps runaway reactions in check. Some producers are exploring catalyst technologies or green chemistry routes, aiming to shrink environmental footprints by using bio-based feedstocks instead of fossil resources for synthesis.
Students learning chemistry in school practice drawing its structure by hand, and that exercise is more than an exam question—it’s an entry point into understanding everything about a material’s future in the world. Methyl acrylate’s formula isn't just trivia; it maps out the path from raw molecule to finished product, with all the responsibility that comes with wide adoption. Every decision, from reaction vessel to worker training, spins out of that one diagram and set of atoms.
Methyl acrylate doesn’t seem like the kind of thing you’d worry about unless you’ve spent some time around chemical plants or labs. I’ve helped set up storage for all sorts of chemicals, and even though the routine sounds simple, mistakes can cause big problems. Anyone who’s dealt with methyl acrylate up close can tell you—getting lax or cutting corners can end up doing real harm.
Breathing in methyl acrylate causes lung irritation. Skin contact burns. It’s flammable, reacts badly with oxidizers, and even the containers can start corroding if the storage space or materials don’t match up. Years ago, I watched a colleague handle a spill—the vapors alone sent two people to the doctor. So, storing it right isn’t just about following a rulebook, it’s about people coming home safe at the end of the day.
Fact is, firefighting methyl acrylate isn’t like throwing water on a campfire. Using the wrong suppression methods spreads the danger. Fire marshals rank it up there with other volatile organics for good reason.
Temperature control matters: Methyl acrylate wants a cozy, cool home—below 25°C (77°F). Leaving drums or containers in a hot warehouse or direct sunlight can lead to unexpected pressure build-up or leaks, and nobody wants to deal with an indoor chemical geyser. Years in warehouse management taught me: humidity wrecks labels, messes with seals, and creeps in everywhere. Keeping things dry and shaded keeps more than just the labels intact.
Ventilation keeps headaches away: Stuffy air gives these fumes a chance to build up, and one spark could turn a storeroom into an emergency. Any place storing this stuff benefits from fans or exhaust systems designed for chemicals, not the basic ones you pick up in hardware stores. Good airflow, maintained filters, and routine checks make all the difference.
No mystery containers: Methyl acrylate eats through certain plastics. Metal drums lined with polyethylene or special polymers handle it best. Local chemical suppliers usually have recommendations, and following those keeps you from swapping emergency calls for cleanup crews. Mixing up containers or ignoring warning labels risks leaks or massive cleanups.
Everyone deserves clear instructions and proper gear. Warehouse managers or technicians often learn by accident—literally. Unlabeled containers, poorly kept logs, or missing spill kits are warning signs. I’ve found that regular training, drills, and straight talk go further than thick manuals. Keeping fire extinguishers for chemical fires within reach, using chemical-rated gloves and eye protection, and marking storage zones makes life simpler for everyone involved.
A system for quick reporting goes a long way. In real-world jobs, people who spot leaks or smells need an easy way to get action fast—no red tape, no waiting. Chemical safety isn’t just company policy, it’s a personal decision to act with care for coworkers and neighbors.
It’s easy to shrug off the extra steps, especially during busy seasons or staff turnover. But the hospitals and cleanup fees from even a small spill leave a bigger mark. Automation and digital logs can keep track of container ages and conditions, alerting staff if anything needs attention. Sensors or alarms that trigger if fumes build up offer peace of mind.
Regulation won’t catch every slip, so constant awareness from the people actually handling methyl acrylate does more to prevent disasters than any document. Shared responsibility, comfortable communication, and real training—those make all the difference.
Methyl acrylate turns up in many places you might not expect. Factories that make paints, adhesives, coatings, and textiles keep this chemical in regular rotation. The smell lands on you before you realize it’s there—pungent and sharp, almost like sharp glue on a summer day in the workshop. Skin contact or that first breath in a closed room triggers a reminder that safety training exists for a reason.
Your hands get slick with methyl acrylate if gloves get left behind. Skin itches, and a chemical burn crops up if you forget to wash off quickly. Redness, rash, and blisters can follow, making it hard to ignore. Breathing fumes without a proper mask or good airflow gives a raw, scratchy throat and stinging eyes within minutes. The stuff doesn't play around—headaches, dizziness, and feeling oddly spaced out set in after longer exposure. That’s how you know the body doesn’t like what’s floating in the air.
Labs run the numbers and find that repeated exposure damages more than just the skin. The lungs can start wheezing or trigger asthma symptoms. Medical evidence points to long-term risks for people working years around these fumes. Animal studies show harm to the liver and kidneys, giving reason to take even “low-level” exposure seriously. The stuff also irritates eyes in a flash—permanent damage isn’t off the table if protection gets skipped.
I remember a plant supervisor who brushed off a small spill on his arm. Less than an hour later, his forearm blistered up. Occupational health told him that methyl acrylate doesn’t need much time to burn through outer skin layers. Another crew member ended up in the hospital after working near a faulty vent hood; she never could explain why her head spun and her chest tightened until the blood tests showed chemical exposure. Others have reported nosebleeds, coughing fits, and swelling around the lips after tough days on the job.
Methyl acrylate gets classified as a hazardous substance in safety data. The Occupational Safety and Health Administration (OSHA) sets exposure limits below 10 parts per million for an 8-hour shift. Acute overexposure sends people to emergency rooms with burns or respiratory distress. Chronic cases link to reduced lung function and slow organ damage. Even the Environmental Protection Agency (EPA) flags this chemical for its ability to pollute air and water, leading nearby communities to worry.
Workplaces can cut these dangers with simple steps. Protective gear keeps skin and lungs shielded—nitrile gloves, face shields, and proper respirators do most of the heavy lifting. Adequate ventilation and air monitoring back up the gear. Washing stations and immediate first-aid kits need to be part of the setup. Regular safety drills remind teams what not to forget.
Anyone managing facilities using methyl acrylate should never treat training as a formality. Transparent reporting, safer alternatives for less risky jobs, and employee input go a long way. Laws exist, but it takes a work culture where people watch out for each other to really keep chemical hazards from slipping through.
| Names | |
| Preferred IUPAC name | methyl prop-2-enoate |
| Other names |
Acrylic acid methyl ester 2-Propenoic acid, methyl ester Methyl propenoate |
| Pronunciation | /ˈmɛθ.ɪl ˈæk.rɪ.leɪt/ |
| Identifiers | |
| CAS Number | 96-33-3 |
| 3D model (JSmol) | `C=CC(=O)OC` |
| Beilstein Reference | 635262 |
| ChEBI | CHEBI:40941 |
| ChEMBL | CHEMBL1080 |
| ChemSpider | 5287 |
| DrugBank | DB04156 |
| ECHA InfoCard | DTXSID7020182 |
| EC Number | 201-185-2 |
| Gmelin Reference | 6709 |
| KEGG | C01782 |
| MeSH | D008766 |
| PubChem CID | 10758 |
| RTECS number | Numero1000 |
| UNII | 4L1PQA76W9 |
| UN number | UN2031 |
| Properties | |
| Chemical formula | C4H6O2 |
| Molar mass | 86.09 g/mol |
| Appearance | Colorless liquid with a sharp, fruity odor |
| Odor | Pungent odor |
| Density | 0.954 g/cm³ |
| Solubility in water | 7.54 g/100 mL (20 °C) |
| log P | 0.739 |
| Vapor pressure | 40 mmHg (20°C) |
| Acidity (pKa) | 23.1 |
| Basicity (pKb) | 7.38 |
| Magnetic susceptibility (χ) | -7.41 × 10⁻⁷ |
| Refractive index (nD) | 1.401 |
| Viscosity | 0.682 mPa·s at 25°C |
| Dipole moment | 1.77 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 116.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -425.25 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1887 kJ/mol |
| Pharmacology | |
| ATC code | M01AE05 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H312, H315, H317, H319, H332, H335, H341, H351 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P272, P273, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P333+P313, P337+P313, P362+P364, P370+P378, P403+P235, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-2-W |
| Flash point | 10 °C |
| Autoignition temperature | 400 °C (752 °F) |
| Explosive limits | 2.8% - 21.6% |
| Lethal dose or concentration | LD50 oral rat 830 mg/kg |
| LD50 (median dose) | LD50 (median dose): 840 mg/kg (rat, oral) |
| NIOSH | MAK77000 |
| PEL (Permissible) | PEL: 10 ppm |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 250 ppm |
| Related compounds | |
| Related compounds |
Ethyl acrylate Butyl acrylate Acrylic acid Methyl methacrylate Ethyl methacrylate |